Random Mutation and Natural Selection Revisited

AUTHOR: Allen MacNeill
SOURCE: Original essay
COMMENTARY: That's up to you...
Promoters of "intelligent design theory" and other forms of creationism often assert that random mutation plus natural selection (RM+NS) are insufficient to explain the diversity of life on Earth. In particular, people like William Dembski assert that RM+NS cannot work fast enough (even given billions of years) to produce the complex living organisms we observe around us.
In so doing, they attack evolutionary theory using a "straw-man argument," because modern evolutionary theory is not limited to RM+NS alone to produce adaptations, nor to explain the diversity of life on Earth. In particular, while there is no empirical evidence that would lead one to believe that mutations are produced by an "intelligent designer," it is also not true that mutations alone must supply the variation necessary for evolution by natural selection.
In particular, while it is true that any given mutation is random (as far as we can tell), a series of mutations which are then preserved as the result of natural selection aren't really random at all, at least not in the way that is often depicted by critics of evolutionary theory. In classical evolutionary theory, as first mathematically formalized by R. A. Fisher, the variation that is necessary for the raw material for natural selection is the result of a large number of individual alleles, all producing variations of the same trait, such as height or skin color in humans. In this model, a normal distribution of heights or skin colors are produced by combinations of different alleles, each influencing some fraction of the overall height, producing what Fisher and others called "continuous variation." Selection then preserved one or a few of the various allele combinations by preserving the individuals that carried the controlling alleles.
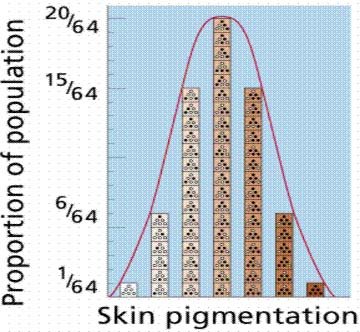
In this model, evolutionary change would necessarily be slow and gradual, as changes in the overall mean value for any trait would require the gradual accumulation of mutations in each of the many alleles that controlled the trait. Since the observable mutation rate is very low (at least, the rate of mutations that significantly affect most phenotypic traits is very low), the argument was that directional change in any given trait was something like a wagon train: only as fast as its slowest constituent. That is, change in the overall distribution of the trait (such as height) depended on the rate of mutation of all of the alleles controlling it, and required that a sufficient proportion of the alleles that were preserved by selection mutate and then be selected in the same "direction" (e.g. for greater height).
However, subsequent field and laboratory investigations into the genetic and developmental control of such variable traits have shown the multiple allele/continuous variation model upon which the "modern synthesis" was based is, in fact, not the way most traits apparently evolve. For example, consider a mutation that causes an increase in size of a particular anatomical feature (e.g. a finch's beak). Most such features are regulated by a set of genes that are themselves regulated by a homeotic gene (or a few such homeotic genes; in the case of Darwin's finches, the controlling homeotic gene is called bmp4, for "bone morphology protein 4") [1]. Homeotic genes, like many but not all genes, do not produce a purely monotonic trait (i.e a trait with no variation). Instead, they produce a trait that varies somewhat between individuals, in what approximates a normal distribution. In the case of finch beaks, this means that in any population of finches, there are some individuals with small beaks, some with large beaks, and most with intermediate beaks. All of these finches could easily have the same allele for the homeotic gene controlling the trait. The variation in beak size would therefore be the result, not of the expression of different alleles, but rather of the different outcomes of the expression of the same allele of the homeotic gene, developing differently in different individuals as the result of a combination of chance and environmental conditions (this is how humans differ in heights, for example).
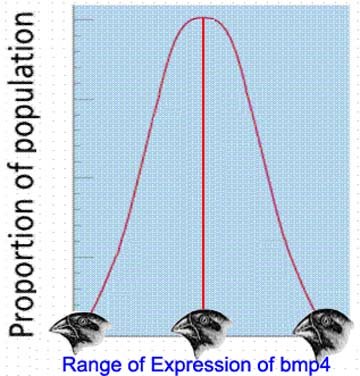
Now consider a situation in which an environmental change (for example, a drought), selected for individual finches with larger beaks. At the level of the controlling homeotic gene, this could mean one of two things: either the larger beaks are still within the developmental limits of the original allele, or another allele (i.e a mutant) has arisen, with an overlapping developmental pattern but a higher mean value for beak size. If the former is the case, then a return to the original environment would result in a return to the original mean beak size.
However, if the latter were the case, then there would be a built-in bias toward finches with larger beaks in the resulting population. This would also mean that the "base" allele - i.e. the new mutant allele - would start out producing a larger mean beak size along with the usual normal distribution of beak sizes. If the environmental change persisted, new alleles might arise, but they would begin with a "norm of reaction" that would produce significantly larger mean beak sizes, along with a normal distribution with significantly larger beaks at the upper tail of the distribution.
In other words, the existing alleles for such a trait would bias subsequent mutations in the "direction" of larger beaks, simply because the pool of potential new alleles would already start out biased in that direction. Therefore, the mutations and developmental changes that were available from one generation to the next would be biased in the direction of whatever phenotypic trait resulted in the highest reproductive success.
This process, called genetic accommodation [2], is part of the new science of evo-devo, which renders much of the classical "evolutionary synthesis" obsolete, and at the same time explains how such phenomena as punctuated equilibria can be integrated into a unified theory of evolutionary development. In particular, genetic accommodation and similar processes can explain how natural selection alone can produce both rapid and directional change in phenotypes over time, thereby making any resort to "intelligent design" unnecessary and irrelevant.
REFERENCES CITED:
[1] Pennisi, E. (2004) Bonemaking protein shapes beaks of Darwin's finches. Science, Vol. 305. no. 5689, p. 1383, available at : http://www.sciencemag.org/cgi/content/summary/305/5689/1383
[2] West-Eberhard, M. J. (2003) Developmental Plasticity and Evolution. Oxford, UK, Oxford University Press. See especially pages 147 to 158.
--Allen
Labels: engines of variation, evolutionary philosophy, genetic mutation, genetic variation, microevolution, natural selection, random mutation




4 Comments:
Superb. I very much enjoy the specific examples you give. It clarifies a lot that is not always made explicit at other science sites.
In particular, while it is true that any given mutation is random (as far as we can tell), a series of mutations which are then preserved as the result of natural selection aren't really random at all, at least not in the way that is often depicted by critics of evolutionary theory.
I believe that it is essential to clarify the nature of the term 'random' when it applies to variation. Random refers to the fact that variation does not arise exclusively beneficial to the environment in question but rather that a mixture of variations arise all the time and that the environment selects for variation that confers a particular benefit to the organism.
Here things get interesting as we are now interested not in evolution itself, but rather evolution of evolution (also known as evolvability). Evolvability is a fascinating concept as it helps understand how and why evolution works. For instance Toussaint has shown how neutrality is an essential aspect of evolvability. Imagine how neutrality both confers robustness as well as evolvability to the genome... Others have shown how neutrality itself is a selectable trait.
So where am I going here? Well, variation may be random with respect to immediate benefits but that does not mean that variation can have no memory of what worked in the past. In fact, if a particular mutation has been very succesful in the past it may be selected for. An example is the hypermutation mechanisms. When under stress the organisms start generating mutations, in the hope of finding a solution for their predicament. So in other words, mutations are random but also are biased as their distribution may reflect the successes in past history.
On pharyngula PZ Myers discussed Evolution of a polyphenism
Yet another example of an evolutionary mechanism. Remind me again, what does ID have to offer here?
Dembski was apparantly not kidding when he stated
As for your example, I’m not going to take the bait. You’re asking me to play a game: “Provide as much detail in terms of possible causal mechanisms for your ID position as I do for my Darwinian position.” ID is not a mechanistic theory, and it’s not ID’s task to match your pathetic level of detail in telling mechanistic stories. If ID is correct and an intelligence is responsible and indispensable for certain structures, then it makes no sense to try to ape your method of connecting the dots. True, there may be dots to be connected. But there may also be fundamental discontinuities, and with IC systems that is what ID is discovering.”
Link
Indeed; by declining to get into the "details," Dembski exhibits his distain for what every scientist knows is the heart of science itself: the "details" of what and how and why things are the way they are.
P.S. PvM, I hope you will be participating in the course blog for the upcoming Cornell Evolution/Design course; the link is:
http://evolutionanddesign.blogsome.com/
Post a Comment
<< Home